The Covid-19 vaccine candidate has been developed under the collaboration between Abu Dhabi-based G42 Healthcare and Sinopharm CNBG
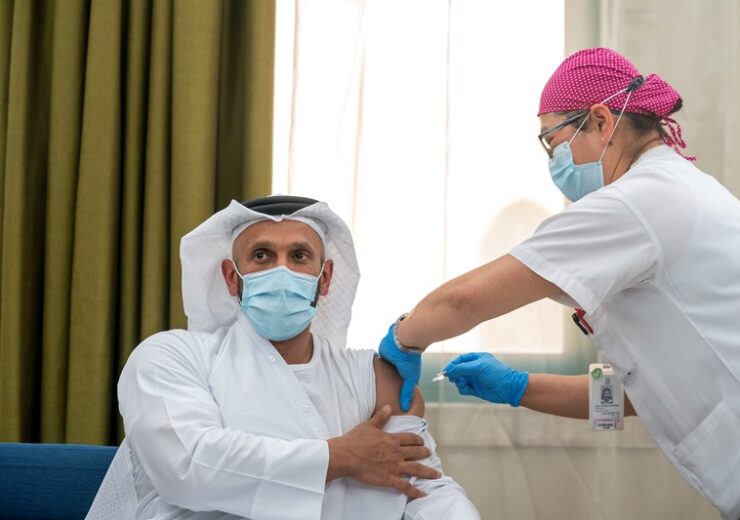
Abu Dhabi begins first Phase 3 clinical trial of inactivated vaccine against Covid-19 (Credit: AETOSWire).
China National Biotech Group (Sinopharm CNBG) has initiated the WHO enlisted global Phase 3 clinical trial of an inactivated vaccine against Covid-19 in Abu Dhabi.
The vaccine candidate is said to be the world’s first inactivated vaccine to enter a Phase 3 clinical trial, and Abu Dhabi department of health chairman Sheikh Abdullah bin Mohammed Al Hamed was the first person to be administered the inactivated vaccine against Covid-19.
The Covid-19 vaccine candidate has been developed under the collaboration between Abu Dhabi-based G42 Healthcare and Sinopharm CNBG.
G42 Healthcare CEO Ashish Koshy said: “We are enormously proud to partner with Sinopharm CNBG in this groundbreaking phase III clinical trial. Using our AI solutions, super-computer, advanced diagnostics solutions for COVID-19, and G42 Healthcare will be responsible for running clinical operations for these trials.”
UAE National Covid-19 Clinical Management Committee Nawal Ahmed Alkaabi said: “Our participation in this trial enables us to make a major contribution in the global fight to combat the Covid-19 pandemic. We are proud to facilitate the testing process that if successful leads to the manufacture of a vaccine.”
Abu Dhabi Health Services (SEHA) is conducting the clinical trial of inactivated Covid-19 vaccine
The UAE has been selected for the clinical trials, as the country shelters more than 200 nationalities, and facilitates the research across multiple ethnicities, increasing its feasibility for global application. Abu Dhabi Health Services (SEHA) is conducting clinical trials and providing clinical facilities.
The UAE Health Authorities have granted a permit for up to 15,000 volunteers to participate in the clinical trials, and G42 Healthcare and SEHA are working towards achieving a minimum of 5,000 participants for the trials being supervised by the Department of Health Abu Dhabi and SEHA
The Phase 3 clinical trial is being conducted according to international guidelines stipulated by WHO and USFDA, following the completion of Phase 1and 2 trials conducted by Sinopharm in China, which is said to have resulted in 100% of volunteers generating antibodies.
Sinopharm CNBG biological products president Jingjin Zhu said: “The UAE is a nation of innovation and tolerance, home to individuals from every part of the world and ethnic background.
“We will work closely with our partner G42 Healthcare, with the support of the Abu Dhabi health authorities to complete these clinical trials successfully and make this vaccine available to people in need worldwide.”
