The FDA approval was based on the findings from the phase 2b VISION-DMD trial in which Agamree met the primary endpoint Time to Stand velocity versus placebo at 24 weeks of therapy with a good safety and tolerability profile
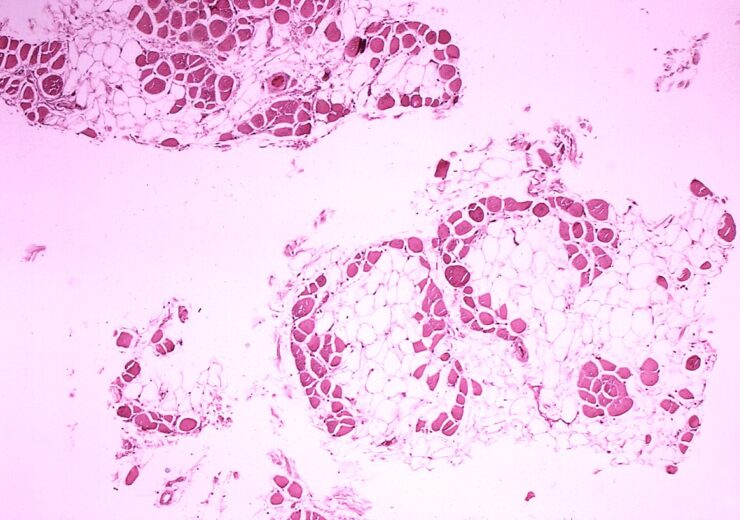
A microscopic image of cross-sectional calf muscle from a DMD patient. (Credit: Dr. Edwin P. Ewing, Jr. from Wikimedia Commons)
Santhera Pharmaceuticals has received the US Food and Drug Administration (FDA) approval for Agamree (vamorolone) to treat certain patients with Duchenne muscular dystrophy (DMD).
The FDA has approved Agamree oral suspension 40mg/ml for DMD patients aged two years and older.
The FDA approval was based on the findings from the phase 2b VISION-DMD trial as supplemented with safety information gathered from three open-label studies, including extension studies.
According to the results, the corticosteroid medication showed equivalent efficacy to the existing standard of care corticosteroids.
It met the primary endpoint Time to Stand (TTSTAND) velocity versus placebo at 24 weeks of therapy and showed a good safety and tolerability profile.
The results indicated a reduction in adverse events, particularly related to bone health, growth trajectory, and behaviour.
The research on the development programme was conducted by Santhera’s partner ReveraGen and 32 academic clinical trial centres in 11 countries.
Santhera CEO Dario Eklund said: “We are delighted to secure FDA approval which comes just weeks after the positive opinion from the CHMP of the European Medicines Agency.
“This is a hugely important moment for DMD patients who need an efficacious and well-tolerated therapy for this debilitating condition.
“Today is a very satisfying day for the Santhera team and we are grateful to the patients, their families and physicians who participated in the vamorolone programme.”
Following FDA approval of Agamree for DMD, Catalyst Pharmaceuticals, the US license holder of the drug, will give Santhera $36m.
This includes a $10m approval milestone to the company and an extra $26m to pay for milestone commitments to contracted third parties.
In addition, Catalyst will absorb Santhera’s related third-party royalties on vamorolone sales in all indications in North America and will pay Santhera sales-based milestones of up to $105m as well as up to low-teen percentage royalties under the terms of the agreement.
Now, Santhera will give its partner Catalyst the US marketing authorisation (NDA) for Agamree.
Catalyst intends to introduce the medicine in the US in the first quarter of 2024.
In Europe, the European Commission is anticipated to approve Agamree for the treatment of DMD in late 2023, in response to the positive opinion from the EMA’s advisory committee in October.
