CAL02 is a non-biological bacterial virulence neutraliser and anti-infective agent intended to treat severe community-acquired bacterial pneumonia as an add-on therapy to standard of care
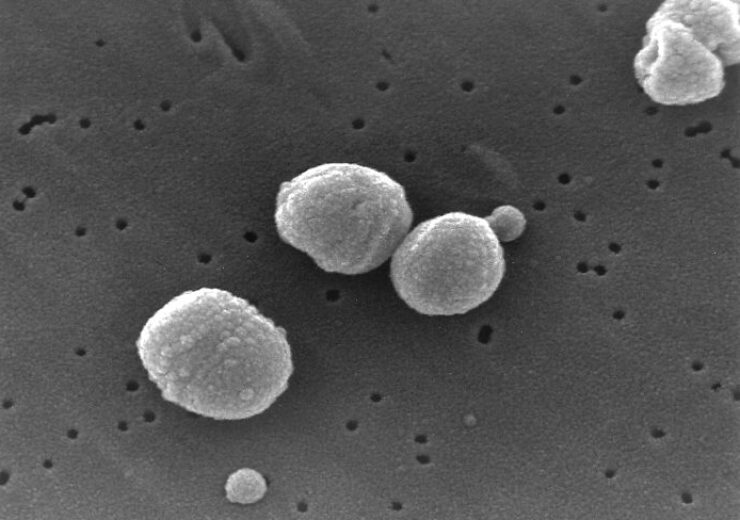
A micrograph of Streptococcus pneumoniae, a common cause of pneumonia. (Credit: CDC/Janice Carr/Wikimedia Commons)
Eagle Pharmaceuticals announced that its CAL02 drug candidate has received a qualified infectious disease product (QIDP) designation under the Generating Antibiotic Incentives Now (GAIN) Act as well as the fast track designation from the US Food and Drug Administration (FDA).
CAL02 is an investigational non-biological bacterial virulence neutraliser and anti-infective agent. It is intended to treat severe community-acquired bacterial pneumonia (SCABP) as an add-on therapy to standard of care.
With QIDP, Eagle Pharmaceuticals anticipates getting five years of marketing exclusivity upon approval or three years without a new chemical entity (NCE) designation.
In total, the firm will get eight or ten years of exclusivity for CAL02 upon new drug application (NDA) clearance.
According to Eagle Pharmaceuticals, the drug candidate acts as a competitive decoy for bacterial virulence factors, which supports infection-related issues like sepsis, septic shock, and death.
CAL02 contains proprietary liposomes made to capture the virulence factors produced by a range of Gram-positive and Gram-negative bacteria that drive infectious diseases such as severe pneumonia.
The US-based pharmaceutical firm has obtained the worldwide exclusive license on CAL02 from Combioxin.
Eagle Pharmaceuticals president and CEO Scott Tarriff said: “Receiving QIDP designation underscores the importance of CAL02 for potentially treating SCABP, and the Fast Track Designation allows us to work even closer with the FDA to bring patients a new treatment option sooner as we would also be eligible to request Priority Review for our application.
“Antibiotics alone, unfortunately, cannot win the war against pneumonia. CAL02 would serve as an add-on to standard of care antibiotic therapy for the prompt treatment of severe bacterial pneumonia and its devastating consequences.
“In an era of increasing resistance to standard therapies, CAL02 represents a potential resistance-free empiric therapy to protect organs and prevent pro-inflammatory cascades leading to severe and fatal outcomes.”
Currently, CAL02 is covered by a US Patent, which lasts until September 2035. The company may apply for a patent term extension that would extend protection for an additional five years, until 2040.
Eagle Pharmaceuticals is planning to conduct a Phase 2 clinical trial to assess the efficacy and safety of CAL02. It will enrol around 276 SCABP patients from all over the world.
