The CONSTRUX Mini Ti System is designed with nanoscale surface features and is said to be the first 3D-printed titanium (Ti) interbody from Orthofix
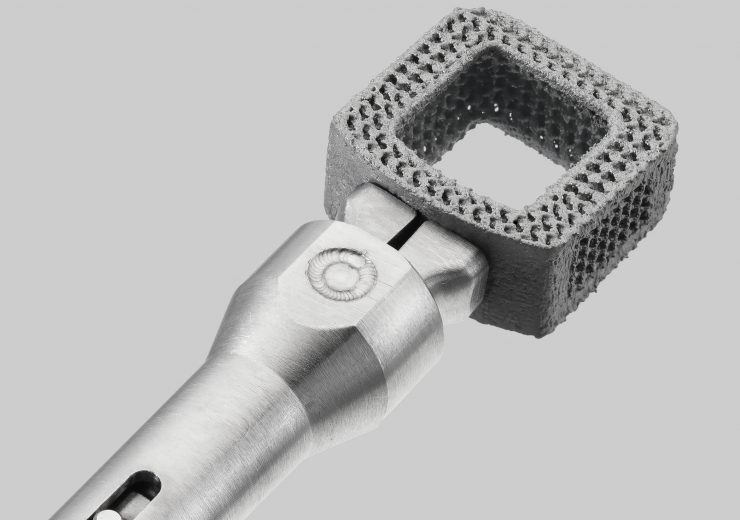
CONSTRUX Mini Ti Cervical Spacer System. (Credit: Orthofix Medical Inc.)
Orthofix Medical has secured the US Food and Drug Administration (FDA) 510(k) approval for its 3D-printed CONSTRUX Mini Ti Spacer System.
The US-based medical device company also announced the first patient implant of its cervical spacer system, developed with Nanovate Technology.
The system has been developed to enhance anterior cervical discectomy and fusion (ACDF) procedures.
Also, the company said that its CONSTRUX Mini Ti cervical spacer system is the first 3D-printed titanium interbody it has introduced to the market.
Loma Linda University Medical Centre orthopaedic spine surgeon Wayne Cheng performed the first patient implant procedure.
Cheng said: “The ACDF procedure, which is often used to treat a herniated cervical disc or degenerative disc disease, involves replacing a patient’s damaged cervical disc with an interbody packed with a biologic to promote fusion in order to provide stability and strength to the area.
“The CONSTRUX Mini Ti System’s optimised porosity and surface allows bone to grow into and through the spacer in order to aid with the patient’s fusion.”
The CONSTRUX Mini Ti Spacer System with Nanovate Technology joins the company’s FDA approved products with nanotechnology.
The products include CONSTRUX Mini PTC Spacer System, Pillar SA PTC Spacer System and the FORZA PTC Spacer System.
With 3D-printed porous titanium with macro, micro, and nanoscale surface, the CONSTRUX Mini Ti Spacer System features endplates with 400micron pores and 50% porosity.
Also, the implant features a large centre opening with concaved inner walls for packing bone-grafting material, and straightforward instrumentation for easy implantation.
Orthofix said that the 3D-printed endplates of the implants with Nanovate Technology are superior to solid PEEK devices.
Implants with Nanovate Technology would significantly increase the growth factors related to osteogenesis and osteoblast maturation, resulting in bone ingrowth, said the company.
Orthofix global spine president Kevin Kenny said: “Orthofix’s cervical spine offerings feature a wide array of implants ranging from motion-preserving products like the M6-C artificial cervical disc to advanced interbody and fixation solutions that aid surgeons in restoring spinal alignment and decreasing pain and nerve compression.
“We are dedicated to expanding our comprehensive cervical spine solutions with technologies like the CONSTRUX Mini Ti Spacer System that can make a meaningful difference in our product offerings and in the lives of patients.”
