The regulatory approval facilitates commercial marketing, sale, and distribution of Patient Specific Talus Spacer Implants in the US
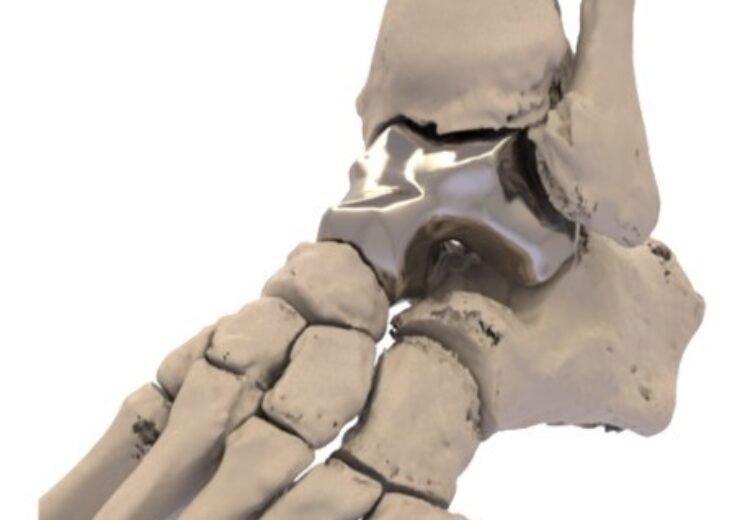
Additive Orthopaedics Patient Specific Talus Spacer. (Credit: PRNewsfoto/Additive Orthopaedics, LLC.)
Additive Orthopaedics has received the US Food and Drug Administration (FDA) Humanitarian Device Exemption (HDE) approval for its Patient Specific Talus Spacer for the treatment of avascular necrosis of the talus.
The US regulatory agency indicated Patient Specific Talus Spacer for Avascular Necrosis (AVN) of the ankle joint.
Additive Orthopaedics claimed that its Patient Specific Talus Spacer is the first and only FDA approved patient specific total talus replacement implant in the US.
Additive Orthopaedics president Greg Kowalczyk said: “Avascular necrosis of the talus is extremely painful and debilitating for these patients.
“Surgical treatment options are below-the-knee amputation or joint fusion, which results in loss of motion of the ankle and can have poor outcomes. The Patient Specific Talus Spacer is another example of how 3D printed devices can improve the standard of care.
“This is a tremendous regulatory win which took significant effort from our team and I want to thank everyone, including the U.S. Food and Drug Administration, who assisted in making this technology commercially available in the domestic market for patients suffering from AVN.”
FDA approved Patient Specific Talus Spacer as a humanitarian use device (HUD), which benefits patients in the treatment or diagnosis of a disease or condition that affects not more than 8,000 US people annually.
Additive Orthopaedics said that its new talus spacer is an additively manufactured, or 3D printed, patient specific implant, designed specifically for each patient using CT image data.
Also, the device helps a patient to regain motion and reduce pain until the time a fusion potentially becomes necessary.
The company is planning to begin marketing and commercial launch of its Patient Specific Talus Spacer in the US, immediately.
FDA Center for Devices and Radiological Health Office of Orthopedic Devices director Capt. Raquel Peat said: “Avascular necrosis of the ankle, while a rare condition, is a serious and potentially debilitating one that causes pain and can lead to inhibited motion of the ankle joint, and in some cases, removal of part of the leg.
“Today’s action provides patients with a treatment option that could potentially reduce pain, retain range of motion of their joint and improve quality of life.”
