ORi to Be Launched in the U.S. at ANESTHESIOLOGY 2023 This Weekend
Masimo ORi™ (Photo: Business Wire)
ORi to Be Launched in the U.S. at ANESTHESIOLOGY 2023 This Weekend
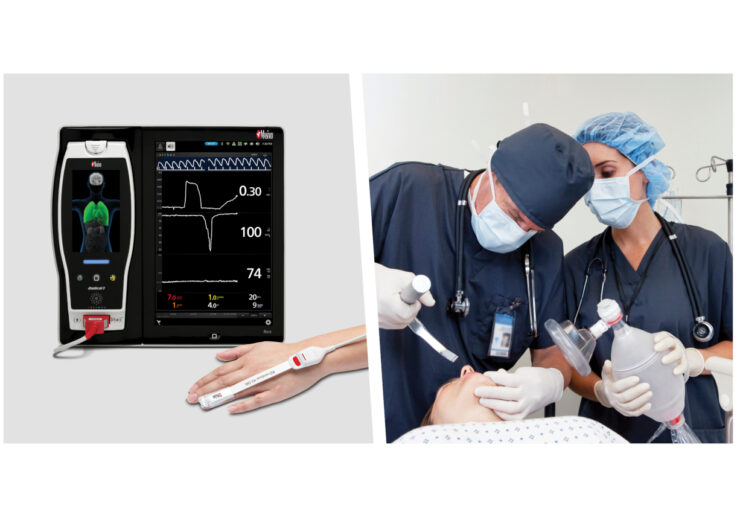
Masimo (NASDAQ: MASI) today announced that ORi™, a noninvasive, continuous parameter designed to provide additional insight into a patient’s oxygen status in the moderate hyperoxic range under supplemental oxygen, has been granted a De Novo by the FDA. Enabled by the multi-wavelength Masimo rainbow® Pulse CO-Oximetry platform, ORi is designed for use in conjunction with oxygen saturation (SpO2) to provide increased resolution of changes in oxygenation under supplemental oxygen. With the De Novo, ORi becomes the first-of-its-kind parameter cleared by the FDA to help clinicians manage oxygen of adults undergoing surgery in perioperative hospital environments.
Without ORi, there is no noninvasive way to monitor oxygenation under supplemental oxygen to manage hyperoxia, or higher than normal oxygenation of arterial blood. There is growing evidence that hyperoxia is harmful and can lead to oxygen toxicity, causing oxygen poisoning or pulmonary tissue damage.1 Currently, clinicians take blood draws that are analyzed to determine PaO2 levels, the partial pressure of oxygen measured by arterial blood gas devices. However, arterial blood analyses are both intermittent and delayed – leaving clinicians blind to the changes in oxygenation occurring between blood draw results.
Masimo ORi addresses these shortcomings by providing continuous insight into the oxygenation of hemoglobin in the moderate hyperoxic range (PaO2 > 100 and ≤ 250 mmHg). ORi is trended continuously with SpO2 as a unit-less index between 0.00 and 1.00 to extend the visibility of the oxygenation of patients beyond SpO2 under supplemental oxygen. By convention, SpO2 is limited to an upper limit of 100%, but oxygenation can rise into hyperoxia when supplemental oxygen is administered. ORi provides clinicians with additional visibility, as a complement to Masimo SET® pulse oximetry, into when oxygenation is increased into, or decreased out of, moderate hyperoxia, in real time.
Numerous studies have demonstrated ORi’s utility. For example, in a study published in Anesthesia & Analgesia of 106 adult patients undergoing scheduled surgery, researchers found decreases in ORi “may provide advance indication of falling PaO2 when SpO2 is still > 98%.”2 In another study published in Intensive Care Medicine, researchers found that the use of ORi monitoring to titrate oxygen rates “allowed an important reduction of the time spent with hyperoxia compared with the use of SpO2 alone,” in a group of 150 mechanically ventilated adult patients randomized to an ORi or a control group.3
Joe Kiani, Founder and CEO of Masimo, said, “Since ORi’s availability and success outside the U.S., perioperative clinicians in the U.S. have been waiting for a way to noninvasively monitor patients under supplemental oxygen beyond the limits of SpO2. We are thrilled that U.S. clinicians can now integrate ORi monitoring – available now on our rainbow SET® platform – into their oxygenation monitoring practices, alongside Masimo SET® Measure through Motion and Low Perfusion™ pulse oximetry, and experience their combined benefits.”
With the De Novo, Masimo is introducing a new sensor line to the market, RD rainbow™ 4λ sensors, expanding the RD family of pulse CO-oximetry sensors, which are now available in four levels of capabilities:
- RD SET®, which utilizes two wavelengths (2 LEDs) and features SET® pulse oximetry.
- RD rainbow 4λ, which utilizes four wavelengths (4 LEDs) and adds the ability to measure ORi.
- RD rainbow 8λ, which utilizes eight wavelengths (8 LEDs) and enables SpHb® (total hemoglobin) monitoring alongside ORi and other measurements.
- RD rainbow 12λ*, which utilizes twelve wavelengths (12 LEDs) and offers 12 total parameters, including SpHb, ORi and fractional oxygen saturation (SpfO2™) for visibility of the impact of dyshemoglobins, SpCO® and SpMet®, on the patient’s oxygenation.
Jesse M. Ehrenfeld, MD, President of the American Medical Association and advisor to Masimo, commented, “I can envision a number of scenarios I encounter in my daily clinical practice as an anesthesiologist where ORi would be invaluable. During patient pre-oxygenation, I often find myself unsure of the adequacy of pre-oxygenation, especially in a patient with a significant cardiopulmonary comorbidity or a patient with diminished oxygen reserve capacity. ORi would solve this problem by giving an easy-to-understand parameter that provides visibility to how oxygenation is changing during the pre-oxygenation process. Additionally, I often find that during the management of a difficult airway, it is never quite clear when to stop, re-establish mask ventilation, and allow the patient to recover. Again, ORi would be very helpful in addressing this issue as it can track the trajectory of the patient’s oxygenation status. I am delighted to see ORi receive FDA clearance. It is not often that new parameters are developed which can actually make a real impact in clinical practice.”
Richard L. Applegate II, MD, Chair of Anesthesiology at Loma Linda University Health, California, stated, “Our studies show that ORi provides advanced detection of low SpO2 events. This additional time may allow modification of airway management, earlier calls for help, or assistance from other providers. Advanced detection of worsening oxygenation is valuable in operative and critical care settings and ORi use has the potential to provide continuous monitoring to detect changes in pulmonary function.”
Ken B. Johnson, MD, Professor of Anesthesiology, University of Utah, commented, “I have been eager for the ORi parameter to be available in the U.S. for a long time as it is a unique innovation with the potential to significantly improve patient care. Observing the ORi trend may help clinicians anticipate hemoglobin oxygen desaturations before they occur. Clinical scenarios where this technology may be useful include airway management that takes longer than expected, prompting rescue breaths before desaturation occurs, and procedural sedation with unanticipated prolonged periods of apnea that can trigger rescue maneuvers before the onset of unwanted desaturation. This index in combination with pulse oximetry shows promise in better managing adverse events related to poor oxygenation and improved patient outcomes.”
ORi is granted a De Novo by the US FDA to be used in patients undergoing surgery as an adjunct to SpO2 for increased monitoring resolution of elevated hemoglobin oxygen saturation levels (e.g., due to administration of supplemental oxygen).
The ORi feature is indicated for the monitoring of hemoglobin oxygen saturation levels in patients 18 years and older (adults and transitional adolescents) on supplemental oxygen during no-motion conditions perioperatively in hospital environments.
*SpfO2 and RD rainbow 12λ sensors have obtained CE Marking. Not available in the U.S.
Source: Company Press Release
