According to Emergent BioSolutions, the expanded access will give people the ability to respond in an opioid emergency, thus saving lives and keeping loved ones and communities safe
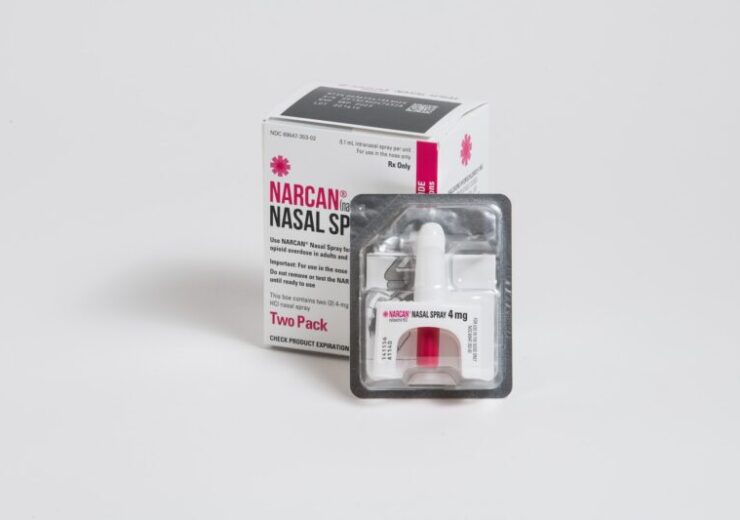
A product shot of Narcan Naloxone HCl Nasal Spray 4 mg. (Credit: NEXT Distro on Unsplash)
American biotech company Emergent BioSolutions has announced the over-the-counter (OTC) availability of the Narcan Naloxone hydrochloride (HCl) Nasal Spray 4 mg for opioid overdose emergency.
The Narcan Nasal Spray is said to be the first US Food and Drug Administration (FDA)-approved OTC 4 mg naloxone product to be available on shelves for opioid overdose.
According to Emergent BioSolutions, the expanded access will give people the ability to respond in an opioid emergency, thus saving lives and keeping loved ones and communities safe.
In case of an emergency, having the NARCAN Nasal Spray on hand or in a first-aid kit can assist in mitigating the effects of opioids while awaiting the arrival of emergency services, said the company.
Emergent BioSolutions products business SVP Paul Williams said: “A steadfast commitment to expanding access to naloxone has always been at the forefront of our work to help save lives and we’re proud to bring Narcan Nasal Spray to many, many more places.
“Expanding availability to online and in-store shelves, increasing awareness and education to reduce stigma, and calling on the public to be prepared, is additive to the current work Emergent does every single day to stem the tide of this public health threat.
“On the eve of International Overdose Awareness Day, we stand by our ongoing efforts with advocates, patients, customers and policy stakeholders to implement efforts that enhance naloxone distribution to all those in need.”
The NARCAN Nasal Spray has the same original prescription strength, device design, and safe and effective 4 mg formulation. It has the potential to reverse the effects of opioids, including fentanyl, the biotech company said.
The life-saving treatment is anticipated to be available for a manufacturer-suggested retail price of $44.99 per carton, or around $22.5 per dose.
The spray, which received FDA approval for OTC treatment of opioid overdose in March, is expected to be available in the US at major mass, drug, supermarket, and internet stores from September 2023.
