The partnership will use Immgenuity's IMTV014 and Biostax's Lodonal and JKB-122 to assess if the immune-modulatory effects of Biostax's pharmacological therapies combined with IMTV014 can result in remission in HIV patients
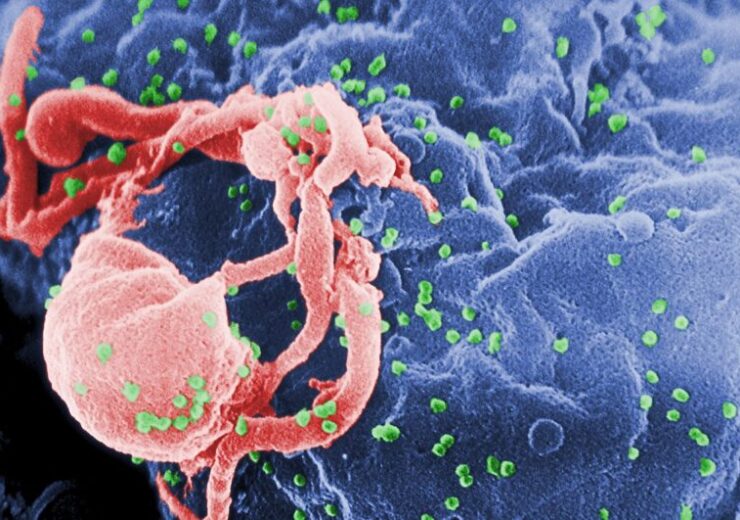
A micrograph of HIV-1 growing from cultured lymphocyte. (Credit: C. Goldsmith from Wikimedia Commons)
Biotechnology company Immune Therapeutics, doing business as Biostax, has signed a research collaboration agreement with US-based Immgenuity to study improvement in Human immunodeficiency virus (HIV) patients.
The partnership will use Immgenuity’s IMTV014 and Biostax’s Lodonal and JKB-122 both independently and jointly to assess if the immune-modulatory effects of Biostax’s pharmacological therapies combined with IMTV014 can result in remission in HIV patients.
According to the terms of the agreement, both firms will work together to accelerate the development of advanced immunotherapies for the treatment of HIV and other diseases.
The partnership is expected to use Biostax’s understanding and experience with using naltrexone as an immune-enhancing and anti-inflammatory agent.
It will also use Immgenuity’s expertise in creating advanced technologies for the identification and characterisation of immune cell populations and mechanisms of immune escape.
The IMTVO14 vaccine platform is an immunotherapy that uses a genetically altered HIV virus that cannot inhibit immune signalling like the original HIV does.
Immgenuity president and CEO Sateesh Apte said: “While HIV treatment has advanced dramatically over the past three decades, people living with HIV still face a lifetime of therapy and chronic illness.
“Remission in HIV remains the ultimate aspiration for both Biostax’s and Immgenuity’s research and development efforts.”
Lodonal, also called low-dose naltrexone, is a rapid-release oral formulation of the drug substance. It can be used as an active immunotherapy drug with anti-inflammatory properties.
It is said to be one of the first drugs approved for use once-a-day as an immune system regulator to manage HIV/ AIDS.
Immune Therapeutics said that previous clinical studies have demonstrated that Lodonal and JKB-122 can improve CD4/CD8 ratios, increase NK cells, and modify the immune system by lowering inflammation and opportunistic infection in HIV patients.
The positive results from these trials will serve as proof that Lodonal and/or JKB-122 can be used along with other treatments to treat HIV patients.
Biostax CEO Noreen Griffin said: “Based upon existing HIV data surrounding both JKB-122 and Lodonal we believe that by performing combined research we will generate additional compelling data, that complements our existing clinical data.
“We are delighted to enter into this partnership that will further develop our product candidates for the treatment of patients with HIV.”
