The FDA has cleared the muscle disorder therapy only for adult LOPD patients whose weight is ≥40kg and who are not improving on their current enzyme replacement therapy
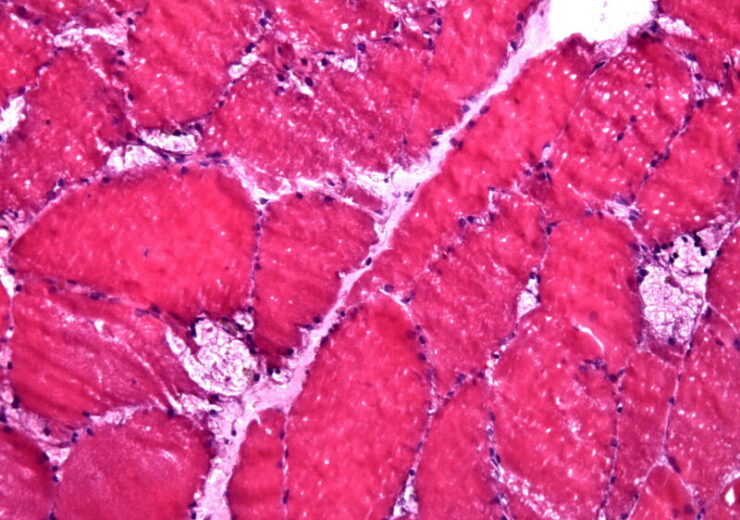
A image of large vacuoles found during muscle biopsy of Pompe disease. (Credit: Jensflorian from Wikimedia Commons)
Amicus Therapeutics has received the US Food and Drug Administration (FDA) approval for its Pombiliti (cipaglucosidase alfa-atga) + Opfolda (miglustat) 65mg capsules to treat late-onset Pompe disease (LOPD).
Pombiliti + Opfolda is a two-component therapy. Pombiliti is a recombinant human GAA enzyme (rhGAA) whereas the company’s oral drug migalastat under the brand name Opfolda is an enzyme stabilizer.
The FDA has cleared the muscle disorder therapy only for adult LOPD patients whose weight is ≥40 kg and who are not improving on their current enzyme replacement therapy (ERT).
Amicus Therapeutics president and CEO Bradley Campbell said: “We are grateful to the Pompe community, particularly the patients, caregivers, families, researchers, and physicians who have contributed to the development process through their commitment to our clinical studies.
“Today’s approval is also a testament to Team Amicus’ extraordinary dedication to patients and our ability to execute on our vision to bring new therapies to the rare disease community.
“Our highly experienced team is ready to launch this medicine in the US, and we look forward to rapidly bringing this new treatment regimen to all eligible adults living with late-onset Pompe disease who are not improving on their current ERT.”
The FDA’s nod was backed by clinical findings found in the late-stage PROPEL study, which is said to be the only study in LOPD to assess ERT-experienced participants in a controlled environment.
The American biotechnology firm said it will launch the two-component therapy in the US with a list price of around $650,000 for a patient weighing about 70kg, reported Reuters. The therapy has been already approved for use in the European Union and the UK in LOPD treatment.
In a related development, Amicus selected Orsini Specialty Pharmacy to dispense Pombiliti and Opfolda in the US.
Orsini Specialty Pharmacy president and CEO Brandon Tom said: “For nearly a decade, Orsini has proudly served the Pompe disease community.
“We are pleased to partner with Amicus to bring Pombiliti and Opfolda to patients suffering from this devastating disease. Together, we aim to make a positive impact on the lives of those affected.”
