Solid tumours, and types of breast and lung cancer look set to be the most common indications for oncology drugs in 2020, according to GlobalData
Chemotherapy drugs, often administered intravenously, are the most commonly used cancer treatment (Credit: NCI)
Cancer trials are set to dominate the field of clinical testing in 2020, says an industry expert.
Some 32% of all drug testing set to take place this year will involve medicines to treat cancer, according to analytics company GlobalData.
About two-thirds of these will be industry-sponsored — funded by a private company working alongside the researcher — while the remainder will be non-industry sponsored.
Among industry-sponsored trials, US pharma company Eli Lilly and Swiss drug firm Novartis are said to be leading the way with the highest number of planned clinical studies in 2020.
After oncology, the biggest research areas are set to be central nervous system disorders such as motor neurone disease (MND), cerebral palsy and epilepsy — which will account for 17.7% of all trials — and infectious diseases including flu, tuberculosis (TB) and the common cold, which will account for 9.8%.
Mohamed Abukar, pharma analyst at GlobalData, said: “In oncology, a majority of the studies are in Phase I and Phase II.
“A large number of early-stage clinical trials within this field are likely to be due to the demand for novel therapeutic approaches addressing unmet medical need, which can be achieved through investigations in the clinical setting.
“This is attributed to the manner in which the burden of cancer worldwide necessitates industry investment to allow for capitalisation on the increasing market size.”
Industry focus on 2020 cancer trials
Seven of the top 10 indications — the reason why a specific drug is being trialled — across the pharma industry will be within the area of oncology.
GlobalData estimates that painkillers and medications for HIV and Alzheimer’s disease are the only drug types in the top 10 not oncology-related.
Solid tumour is the highest of these cancer-related areas, accounting for 3.77% of all drugs being trialled — followed closely by types of breast and lung cancer.
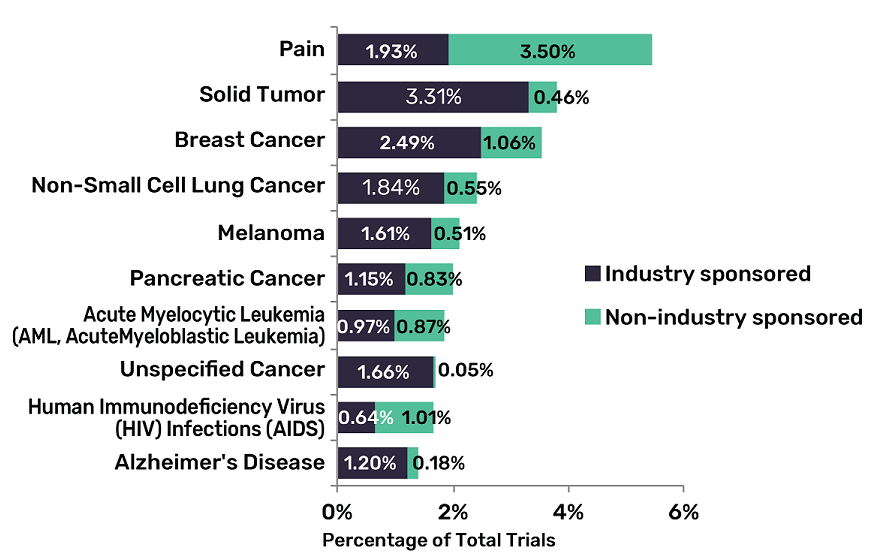
Planned clinical trials across the top oncology indications are predominately industry-sponsored, which “highlights the overall dominance of industry” within early-stage clinical investigations for oncology, according to GlobalData.
The US dominates the research space for planned trials set to be completed in 2020 — accounting for almost the same number as the rest of the top 10 combined.
China holds second place, followed by Australia, France, Canada and the United Kingdom.
