
NS Healthcare is using cookies
We use them to give you the best experience. If you continue using our website, we'll assume that you are happy to receive all cookies on this website.
ContinueLearn More X
We use them to give you the best experience. If you continue using our website, we'll assume that you are happy to receive all cookies on this website.
ContinueLearn More XPress
Release
The attention to a cleaner and safer medical environment is growing and influencing practices globally. In endoscopy, cross-contamination of endoscopes remains one of the top 10 Health Technology Hazards1,2. Bedside cleaning, also called point-of-use precleaning, is a critical process to ensure the efficacy of the subsequent endoscope High-Level-Disinfection. The current ready-to-use cleaning adapters in the market feature the air-water channel cleaning only, not addressing the need for biopsy port cleaning, which is fully exposed to microorganisms during the procedure. In response to this concern and aiming for better practice, GA Health has developed designed a biopsy port adapter for removing initial bioburden immediately after the procedure.
Why should the biopsy port be immediately cleaned after use with the suction and air-water channels?
“Failure to remove foreign material from the outside and the inside of the endoscope can interfere with the effectiveness of subsequent disinfection and/or sterilisation.”3
The biopsy port allows the insertion and withdrawal of collected samples by a biopsy instrument that has direct contact with patients. The extended channel of it is shared with the suction channel. During the procedure, this area is fully exposed to contaminants. Bacteria can attach to the endoscope surface in a matter of seconds or minutes, leading to irreversible attachment5.
Therefore, after the procedure, the biopsy port should be immediately cleaned to reduce the level of biofilm formation. Biofilm can bring challenges to the success of the endoscope reprocessing and eventually cause cross-contamination.
GA Health’s Andorate biopsy port cleaning adapter is designed to draw detergent into the channel for flushing. It can optimise the bedside cleaning process by removing initial contaminants and preventing biofilm formation in the endoscope channel4. It is ready to use, requiring no reprocessing. Additionally, it helps improve the field’s hygiene by reducing the risk of cross-contamination between the procedure and cleaning rooms.
GA Health offers 5 different varieties of cleaning devices. The products are designed to work with Olympus and Fujifilm 700 Series GI endoscopes. To further streamline the process in the hospitals and clinics, these products can be provided sterile with procedure valves as well.
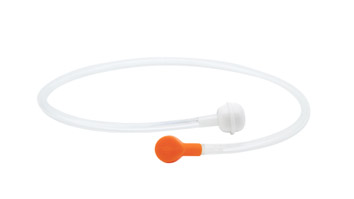

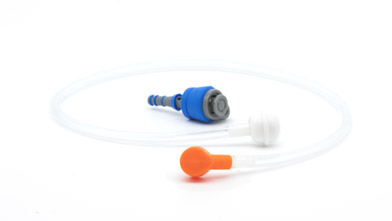


About GA Health
GA Health is a leading infection prevention product developer and manufacturer with global operations in Hong Kong and Ireland. The company was founded by Ken McCabe in 2003 with the vision of becoming the market leader in infection prevention.
GA Health has become a solution provider in endoscopy infection prevention with a wide range of innovative products covering procedures, cleaning, transport and storage.
The company distributes its brand Andorate to 20 countries across Europe, America and Australasia. Moreover, it continues developing products that meet market needs by working closely with our healthcare professionals.
For more information, visit www.gahealth.com
Date of Publication: 8th March 2023
Contact Information: Product@GAHealth.com
1: “Excellence in Scope Reprocessing” session at 2016 SGNA Conference by Laura H. Schneider, RN CGRN CASC of AMSURG Corporation
2:“Is That Scope Really Clean?” session at 2016 SGNA Conference by Barbara Zuccala, MSN RN CGRN of The Valley Hospital 3: “PreventingCross-Contamination in Endoscope Processing: FDA Safety Communication” FDA 2009
3: Pasquale L, Maurano A, Cengia G, Carrara PDM, Germanà B, Graziani MG, Manes G, Pisani A, Golia M, Marciano E, Rodella L, Schiffino L, Gandolfo C, Terrosi C, Cusi MG, Infection prevention in endoscopy practice; comparative evaluation of reusable vs single use endoscopic valves, Infection Prevention in Practice, https://doi.org/10.1016/j.infpip.2021.100123.
4. Gonzalez J A, Vanzieleghem T, Dumazy A et al. On-site comparison of an enzymatic detergent and a non-enzymatic detergent-disinfectant for routine manual cleaning of flexible endoscopes. Endosc Int Open. 2019;7: E412–E420. [PMC free article] [PubMed] [Google Scholar]
5.Alfa, M. J., Sepehri, S., & Olson, N. (2012). Establishing a clinically relevant bioburden benchmark: A quality indicator foradequate reprocessing and storage of flexible gastrointestinal endoscopes. American Journal of Infection Control, 40(3), 233-236.
Alfa, M. J. (2015). Monitoring and improving the effectiveness of cleaning