Philips’ new patient monitoring solutions will support infection-control protocols and remotely provide critical patient information for caregivers
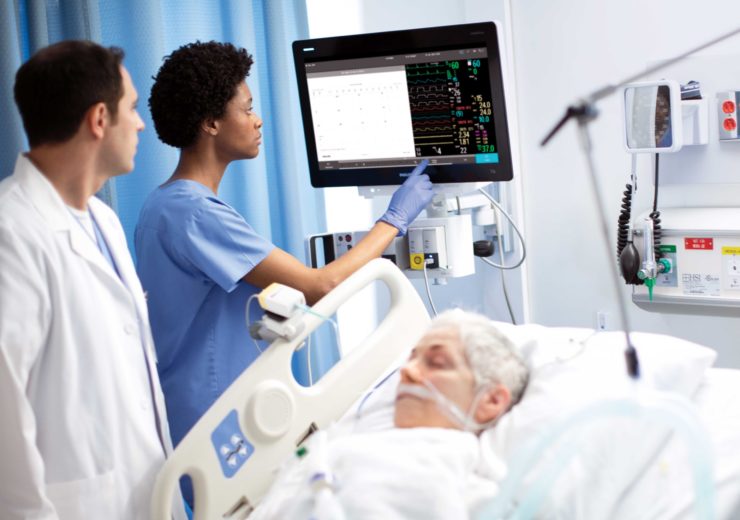
Philips IntelliVue MX850. (Credit: Koninklijke Philips N.V.)
Royal Philips has received the US FDA emergency use authorisation (EUA) for its IntelliVue Patient Monitors MX750/MX850 and IntelliVue Active Displays AD75/AD85, for use during the Covid-19 health emergency in the US.
The health technology company said that its new patient monitoring solutions will support infection-control protocols and remotely provide critical patient information for caregivers, which is much needed while caring for hospitalised Covid-19 patients.
Philips monitoring and analytics general manager Peter Ziese said: “As the world continues to battle against Covid-19, we’re committed to ramping up production of all critical solutions that can help in this time of crisis.
“This FDA EUA for our MX750 and MX850 monitors and IntelliVue AD75 and AD85 Active Displays allows us to do that for these remote patient monitoring solutions, which are of vital need in the ICU.
“At Philips, being able to provide the right information at the right time to caregivers has always been a top priority. Now more than ever, there’s an urgent need to make sure those on the frontline have all the available resources at their disposal.”
The Philips’ patient monitoring solutions have been CE mark approved in 2019
Philips’ IntelliVue Patient Monitors MX750/MX850 and IntelliVue Active Displays AD75/AD85 have been granted CE mark approval in 2019 and are already being used in hospitals across Europe.
The current regulatory authorisation allows the company to start delivering the new remote patient monitoring solution to US hospitals, and help the company in filing for FDA 510(k) approval in the course of 2020.
Philips said that while countries across the globe are gradually resuming elective care to fight against Covid-19, it is increasing the patient monitor production capabilities to address the demand for Intensive Care Unit (ICU) capacity.
In addition, the company said that its IntelliVue Patient Monitors MX750/MX850 and IntelliVue Active Displays AD75/AD85 will provide advanced functionality and clinical decision support capabilities, including Philips’ IntelliVue Horizon Trends information view.
Philips’ IntelliVue Horizon Trends information view shows deviations in vital signs to contextualise a patient’s condition, while also supporting infection-control protocols and access to key information both remotely and at the bedside.
Furthermore, Philips’ Alarm Advisor and Alarm Reporting will help reduce alarm fatigue for caregivers, while the smooth glass surfaces, rounded edges and special surface material of the monitors and displays facilitates cleaning and disinfection.
