The model avoids patients from exposure to high doses of radiation and nephrotoxic contrast agents
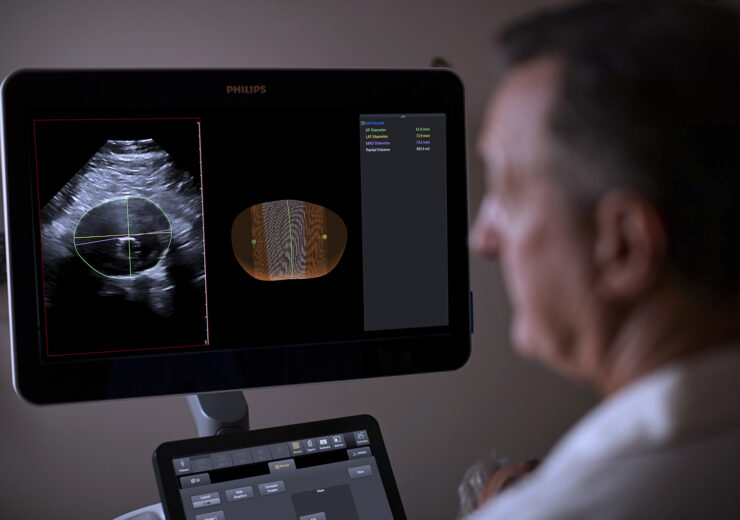
3D ultrasound-based Philips AAA Model. (Credit: Koninklijke Philips N.V.)
Global health technology firm Royal Philips has introduced its Abdominal Aortic Aneurysm (AAA) Model, to provide a more patient-friendly solution than the current standard of care to manage AAA patients.
The company said that its Philips AAA Model is developed based on 3D ultrasound technology.
Also, Philips AAA Model will provide clinicians with accurate diagnostic information, avoiding patients from exposure to high doses of radiation and nephrotoxic contrast agents.
Philips senior vice president and ultrasound general manager Bich Le said: “Regular surveillance of abdominal aortic aneurysm patients is essential, but today’s standard of care has downsides.
“Philips Abdominal Aortic Aneurysm (AAA) Model seamlessly integrates leading Philips technologies including Philips Premium Ultrasound System (EPIQ Elite), Philips Array Transducer (X6-1 xMATRIX) and innovative software into a single solution, to help improve the experience and outcomes for clinicians and patients alike.”
Philips AAA Model granted CE mark in Europe and US FDA approval
AAA is an aneurysm that forms in the lower part of the aorta, and is identified incidentally during abdominal imaging exams but, in some cases, remain undetected until they rupture.
Ruptured AAAs are associated with 80% mortality rate, and underlines the importance of routine surveillance.
Philips AAA Model is developed by integrating advanced software and Philips 3D ultrasound technologies into a single solution, to enhance the diagnostic confidence and improve patient experience.
The software would automatically segment and quantify the size of the aneurysm sac for surveillance of known native, and post-EVAR AAAs.
The current standard of care for AAA includes 2D ultrasound and computed tomography angiography (CTA). Both the modalities have drawbacks, including inter-operator variability and patient exposure to radiation and nephrotoxic contrast agents with CTA.
According to a recent clinical study, 3D ultrasound examination for native AAA surveillance showed a superior inter-operator reproducibility to that of 2D ultrasound.
The clinical study results support the wide use of 3D ultrasound in standard AAA surveillance programmes, the company said.
Philips AAA Model has been granted CE mark for sale in Europe and is approved by the US Food and Drug Administration (FDA) for sale in the US.
