LumiraDx claims that its microfluidic technology and point of care platform supports a wide range of tests with lab-quality performance, at a low cost
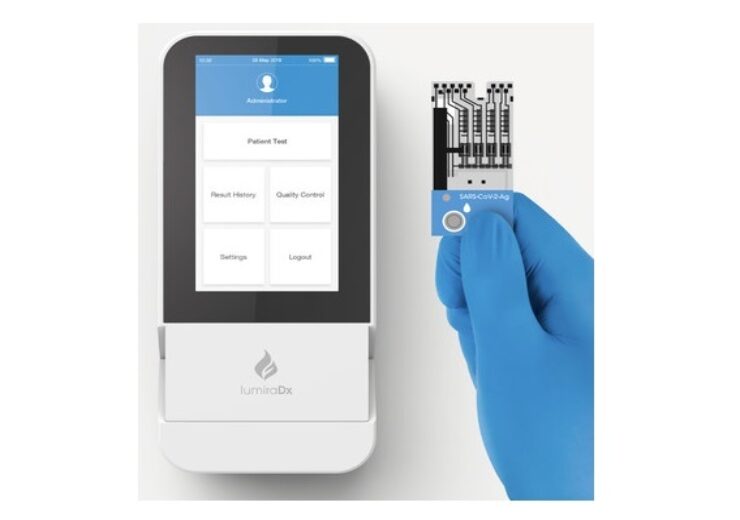
LumiraDx Platform and SARS-CoV-2 Antigen Test. (Credit: LumiraDx)
LumiraDx has received India’s Central Drugs Standard Control Organisation (CDSCO) approval for emergency use of its SARS-CoV-2 Antigen test in the country.
The SARS-CoV-2 Antigen test is a microfluidic test that runs on the LumiraDx point of care Platform to detect antigen nucleocapsid protein from a nasal swab.
It provides results within 12 minutes from the sample application, said the company.
LumiraDx point of care Platform is said to scale down and integrate techniques, which are used in laboratory analysers, to deliver lab-quality diagnostic tests using a single point of care device.
It comes with a small, portable instrument, microfluidic test strip, standardised workflow, and secure digital connectivity to the cloud and hospital IT systems, said the company.
LumiraDx chief commercial officer David Walton said: “The mission of LumiraDx is to transform community-based healthcare through POC diagnostics and make lab-comparable tests accessible to all.
“We are proud to now have a presence in one of the world’s fastest-growing economies and have the opportunity to partner with local health systems and businesses across the country to provide highly accurate and rapid testing.”
In clinical studies, the SARS-CoV-2 Antigen test has shown 97.6% positive agreement and 96.6% negative agreement with the PCR test for patients within the first 12 days of symptoms.
The test has received the US Food and Drug Administration (FDA) emergency use authorisation in August 2020 and CE Mark in September 2020.
LumiraDx India general manager Yogesh Singh said: “There is a significant need for high-quality, accurate point of care testing across India to meet not only the current demand with Covid-19, but also to provide testing for a number of health conditions beyond the pandemic.
“Launching the LumiraDx Platform and microfluidic technology, first with the SARS-CoV-2 Antigen test, will provide next-generation POC testing for patients in rural, urban and semi-urban health settings in India enabling healthcare providers to reduce the impact of acute and chronic diseases across the country.”
LumiraDx said that its platform is being used by the National Health Service (NHS) and Boots in the UK, CVS Pharmacy in the US, and other institutions worldwide.
Also, its test is being deployed, in partnership with the Bill & Melinda Gates Foundation, in several African countries where access to high-quality diagnostics is limited.
