Clinuvel has designed SCENESSE to increase pain -free light exposure in adult patients with phototoxic reactions from erythropoietic protoporphyria (EPP)
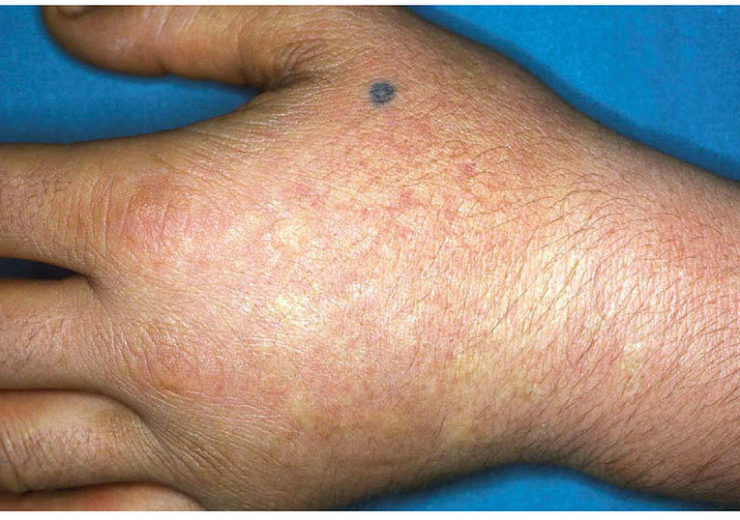
Image: Image showing acute photosensitivity reaction in EPP. Photo: Courtesy of M Lecha, H Puy, JC Deybach/Wikipedia.
Australia-based biopharmaceutical company Clinuvel has secured the US Food and Drug Administration (FDA) approval for SCENESSE (afamelanotide 16mg) for the treatment of erythropoietic protoporphyria (EPP) patients in the US.
SCENESSE marks the first systemic photoprotective drug approved as a treatment for EPP, a rare genetic metabolic disorder which causes absolute intolerance to light.
Clinuvel said that its new drug has been indicated to increase pain-free light exposure in adult patients with a history of phototoxic reactions from EPP.
Clinuvel chief scientific officer Dennis Wright said: “Today is a memorable day and victory for EPP patients, their families, and the global medical community who have all supported the development and US approval of the first ever treatment for this debilitating condition and the world’s first systemic photo protective drug.
“EPP patients are born with the disease and need to avoid all sources of light exposure, including sunlight throughout their life, or risk incapacitating burns. SCENESSE is a novel drug and formulation, developed by CLINUVEL to provide photo protection for EPP patients, enabling patients to expose to light, providing them a freedom they never had.”
SCENESSE creates a physical barrier to visible and invisible light in EPP patients
EPP is an inherited metabolic disorder that causes lifelong phototoxicity due to the accumulation and storage of a biological compound, protoporphyrin IX (PPIX), in the blood and tissues.
PPIX is activated when the patient is exposed to visible light and near-visible ultraviolet radiation and causes damage to the surrounding tissue.
The company estimates that there are 5,000 to 10,000 EPP patients across the world. Patients affected by EPP experience severe burning pain underneath the skin, which lasts for days or weeks, forcing them to avoid further exposure to light.
The new drug, SCENESSE, binds to the melanocortin-1 receptor on skin cells and initiates a series of cellular events to activate the melanin pigment and provide a physical barrier to visible and invisible light in EPP patients.
Clinuvel said that SCENESSE was approved by the European Medicines Agency as an orphan medicinal product for the inhibition of phototoxicity in adult patients with EPP in 2014 and was launched in Europe in 2016.
Clinuvel chief executive officer Philippe Wolgen said: “On this day, there are many professionals, patient organisations and stakeholders to be grateful to, but I should not forget the FDA’s Divisional Director who led the review of SCENESSE in EPP. Our team is granted very little time to celebrate and now needs to shift its focus to facilitating drug product access for US EPP patients.”
