The lebrikizumab Phase 3 study programme is designed to confirm the safety and efficacy of lebrikizumab in treating patients with moderate-to-severe atopic dermatitis
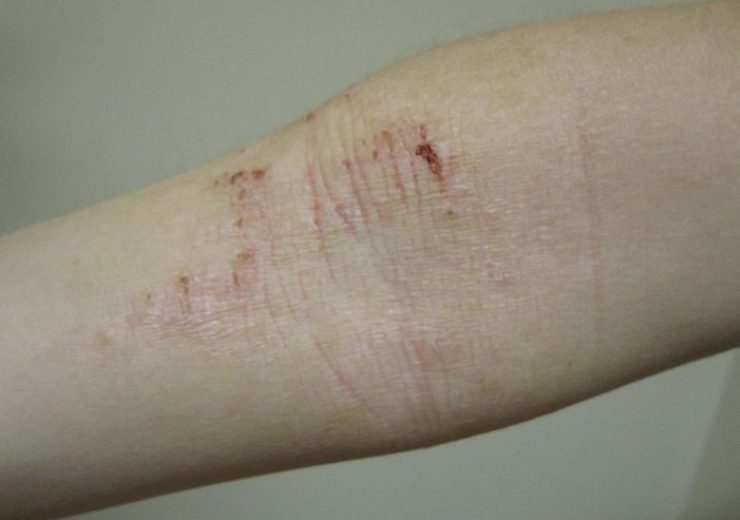
Image: Atopic dermatitis of the inside crease of the elbow. Photo: Courtesy of James Heilman, MD/Wikipedia.
US-based biopharmaceutical company Dermira has dosed the first patient in a Phase 3 study evaluating the safety and efficacy of lebrikizumab in treating atopic dermatitis.
Dermira said that it develops new therapies for chronic skin conditions, and its lebrikizumab is indicated for adult and adolescent patients, aged 12 and older, with moderate-to-severe atopic dermatitis.
Dermira chairman and chief executive officer Tom Wiggans said: “The positive results of our Phase 2b dose-ranging study suggest specifically targeting IL-13 with lebrikizumab has the potential to deliver a best-in-disease therapy for people living with moderate-to-severe atopic dermatitis.
“Our Phase 3 clinical program is designed to confirm those findings and hopefully bring an important new treatment option to the millions of people living with this chronic and often debilitating disease. We expect to report findings from the 16-week induction period of the monotherapy studies in the first half of 2021.”
Lebrikizumab inhibits the biological effects of IL-13 to prevent atopic dermatitis
Atopic dermatitis is the most common and severe form of chronic inflammatory condition ‘eczema’ and a moderate-to-severe form of the disease is characterised by rashes on the skin that often cover much of the body, along with intense, persistent itching.
Atopic dermatitis potentially limits the daily activities and health-related quality of life in patients, with a negative impact on mental and physical functioning.
IL-13 is thought to be a central pathogenic mediator that supports the pathophysiology of atopic dermatitis by promoting type 2 inflammations and causes effects on tissue, targeting skin barrier dysfunction, itch, skin thickening and infection.
Dermira said that lebrikizumab is an investigational, monoclonal antibody that binds IL-13 with very high affinity to specifically prevent the formation of the IL-13Rα1/IL-4Rα heterodimer complex, and subsequent signalling, inhibiting the biological effects of IL-13.
The lebrikizumab Phase 3 study programme is designed to be conducted with two identical, randomised, double-blind, placebo-controlled, parallel-group to confirm the safety and efficacy of lebrikizumab as monotherapy in patients with moderate-to-severe atopic dermatitis.
The studies intend to enrol a total of 800 adult and adolescent patients aged 12 years and older with moderate-to-severe atopic dermatitis at approximately 200 sites in the US, Europe and Asia.
