BinaxNOW Card is a portable rapid Covid-19 antigen test, designed to be around the size of a credit card, and delivers the test results within 15 minutes
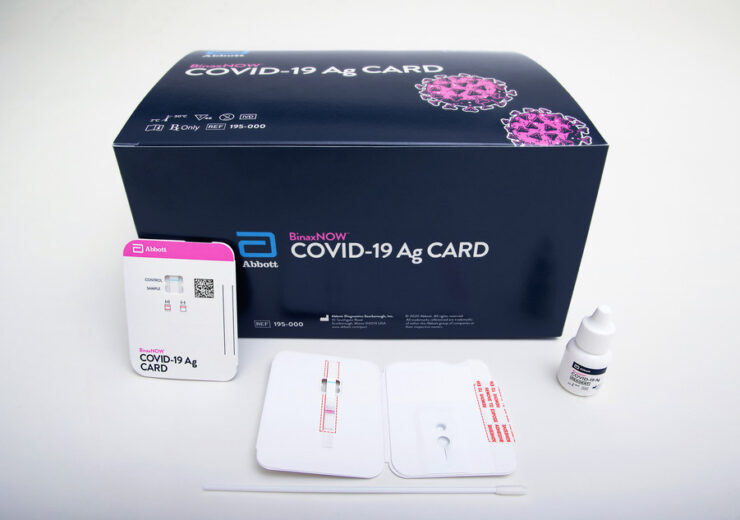
Abbott's BinaxNOW COVID-19 Ag Card is a rapid testing tool. (Credit: Abbott.)
Abbott has received the US Food and Drug Administration (FDA) Emergency Use Authorisation (EUA) for its BinaxNOW COVID-19 Ag Card rapid test to detect Covid-19 infection.
The medical devices company said that its BinaxNOW is a portable rapid Covid-19 antigen test, designed to be around the size of a credit card, and delivers the test results within 15 minutes. The company intends to sell the new test for $5 each.
The new test leverages the company’s lateral flow technology to enable continuous mass testing, and the device does not require any additional equipment, ensuring rapid identification of people with infections to prevent the spreading of the virus.
Also, the company has developed a new mobile app, dubbed NAVICA, to enable people to display the date and results of the test in iPhone and Android devices.
Abbott president and chief executive officer Robert Ford said: “We intentionally designed the BinaxNOW test and NAVICA app so we could offer a comprehensive testing solution to help Americans feel more confident about their health and lives.
“BinaxNOW and the NAVICA app give us an affordable, easy-to-use, scalable test, and a complementary digital health tool to help us have a bit more normalcy in our daily lives.”
FDA authorisation allows use of BinaxNOW Card in point-of-care settings
The FDA EUA allows the use of new BinaxNOW Card by the healthcare professionals in point-of-care settings, qualified for testing and are operating under a Clinical Laboratory Improvement Amendments (CLIA) certification.
The new test will be used as the first line of defence to identify people who are currently infected and who should isolate themselves to help prevent the spread of the disease.
Abbott has designed the test to detect the nucleocapsid protein antigen from SARS-CoV-2 in nasal swabs from individuals suspected of Covid-19 within the seven days from the onset of symptoms.
NAVICA mobile app is an easy-to-use tool that allows people to store, access and display their test results as proof of testing before entering the organisations. The app displays a digital health pass for patients who tested negative.
Ford added: “While BinaxNOW is the hardware that makes knowing your Covid-19 status possible, the NAVICA app is the digital network that allows people to share that information with those who need to know.
“We’re taking our know-how from our digitally-connected medical devices and applying it to our diagnostics at a time when people expect their health information to be digital and readily accessible.”
