The molecular testing will identify the people infected with the virus while antibody tests will determine if a person was previously infected
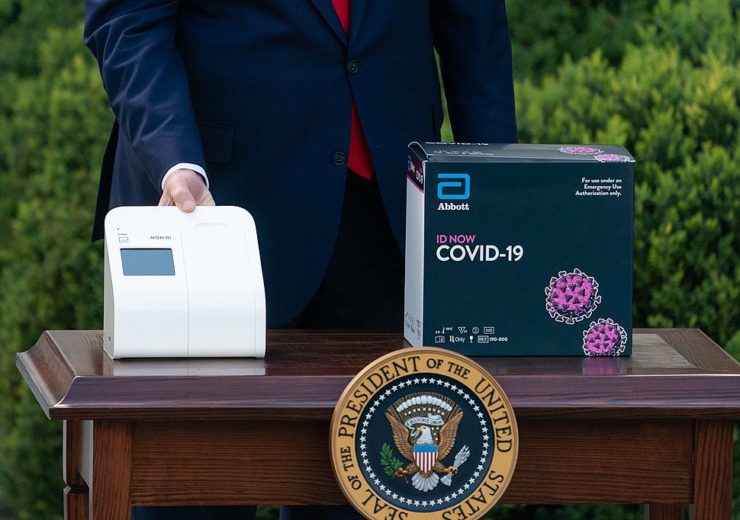
US President Donald Trump displays Abbott’s ID NOW molecular COVID-19 testing kit. (Credit: The White House from Washington, DC/Wikipedia.)
Abbott has launched a new lab-based serology blood test to detect the IgG antibody, thereby identifying the presence of the novel coronavirus (COVID-19) in people.
The new SARS-CoV-2 IgG test will identify the IgG antibody, which is a protein that the body produces in the late stages of infection and may remain for up to months and possibly years after a person has recovered.
Abbott president and chief executive officer Robert B Ford said: “We continue to contribute in a significant and meaningful way by providing new solutions across our diagnostics testing platforms. I’m extremely proud of the many Abbott people who are working around the clock to get as many tests as we can to healthcare workers and patients.”
Abbott antibody test will expand the COVID-19 testing and help healthcare workers
According to the company, antibody testing is an important next step to identify someone who has been previously infected and will provide more understanding of the virus, including the capability of antibodies to stay in the body and immunity they provide.
Molecular testing will identify the people infected with the virus, while antibody tests will determine if a person was previously infected.
Abbott said that the new SARS-CoV-2 IgG test is its third COVID-19 test, and adds to its existing COVID-19 tests including m2000 molecular laboratory system and ID NOW molecular point-of-care device.
The company is initially offering its IgG antibody test on its ARCHITECT i1000SR and i2000SR laboratory instruments, under the US Food and Drug Administration (FDA) notification without an Emergency Use Authorization (EUA) pathway outlined in Policy for Diagnostic Tests for COVID-19.
Abbott is also planning to file an EUA submission with the FDA and CE Mark to the IVD Directive (98/79/EC) in the European Union.
The company is ramping up manufacturing for antibody testing and intends to immediately ship approximately one million tests to the US this week.
